Enhancing Electrochemical Hydrogen Evolution Efficiency via UV Ozone Treatment: 3D Electrode Carbon Cloths with Defect-Enriched MoS2
Leonardo Togar Samosir, Ferry Anggoro Ardy Nugroho, Vivi Fauzia
Electrochimica Acta 2024, 496, 144483
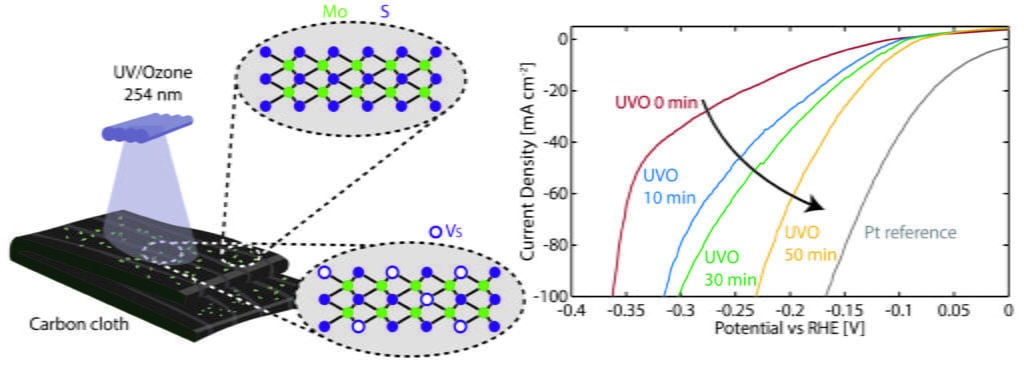
Electrochemical water splitting, a key process for extracting hydrogen energy from water, is recognized as a promising eco-friendly technology. Molybdenum disulfide (MoS2), especially when possessing abundant defects, emerges as a cost-effective and efficient catalyst for hydrogen evolution reaction (HER), presenting a viable alternative to noble metals such as platinum (Pt). However, the impact of UV-Ozone (UVO) in generating defects in bulk 2H-MoS2 remains underexplored. Here, we successfully synthesized 2H-MoS2 using the hydrothermal method on a 3D conductive carbon cloth. Subsequently, the sample underwent UVO treatment for three different durations of 10, 30, and 50 min. Our results demonstrate that a 50-minute exposure to UVO yields the most effective HER performance, with a significant decrease in the onset potential from 189 mV to 116 mV and a low Tafel slope of 64 mV/decade. This enhancement is attributed to increased surface activity, evident in a more than fourfold increase in double-layer capacitance (Cdl). Importantly, the 50-minute UVO treatment not only significantly improves the purity of MoS2 and increases the number of edge sites, but also lead to an increase in sulfur vacancies and the formation of amorphous oxygen-incorporated MoSx. These edge sites, along with sulfur vacancies and oxygens incorporation, are considered ideal for hydrogen binding, enhancing electron transport, and improving HER activity.
